System Biosciences
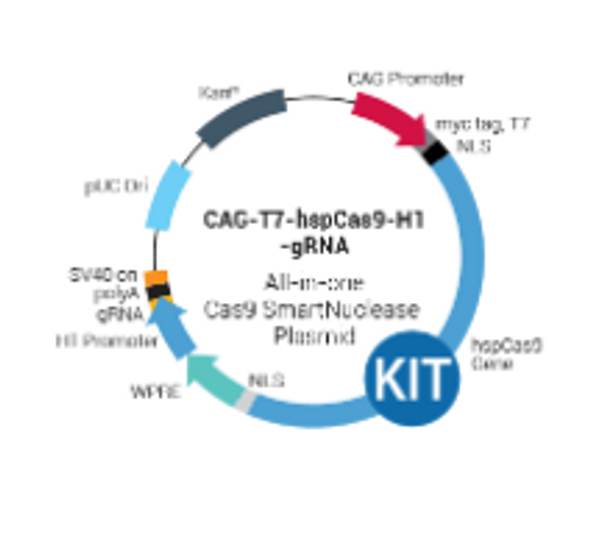
CAG-hspCas9-H1-gRNA SmartNuclease vector
- SKU:
- CAS920A-1
- Availability:
- Usually Shipped in 5 Working Days
- Size:
- 10 ug
- Shipping Temperature:
- Blue Ice
Description
CAG-hspCas9-H1-gRNA SmartNuclease vector. Cat# CAS920A. Supplier: SBI System Biosciences
- Conveniently deliver Cas9 SmartNuclease and gRNA with a single vector
- Reduce off-target activity
- Drive Cas9 expression with the CAG promoter, which provides high expression levels in in primary cells and stem cells
- Express gRNA from the H1 promoter for maximum specificity and choice of targets
- Ensure efficient import of Cas9 SmartNuclease to the nucleus with N-term and C-term nuclear localization signals (NLSs)
Products

Overview

- Conveniently deliver Cas9 SmartNuclease and gRNA with a single vector
- Reduce off-target activity
- Drive Cas9 expression with the CAG promoter, which provides high expression levels in in primary cells and stem cells
- Express gRNA from the H1 promoter for maximum specificity and choice of targets
- Ensure efficient import of Cas9 SmartNuclease to the nucleus with N-term and C-term nuclear localization signals (NLSs)
- Boost Cas9 Nuclease gene expression and stabilize the transcript via the WPRE regulatory element after the C-term NLS
- Easily detect and/or purify the Cas9 SmartNuclease protein with the N-term myc-tag

How It Works
Genome engineering with CRISPR/Cas9
For general guidance on using CRISPR/Cas9 technology for genome engineering, take a look at our CRISPR/Cas9 tutorials as well as the following application notes:
CRISPR/Cas9 Basics
Through careful selection of the target sequence and design of a donor plasmid for homologous
recombination, you can achieve efficient and highly targeted genomic modification with CRISPR/Cas9.
The system
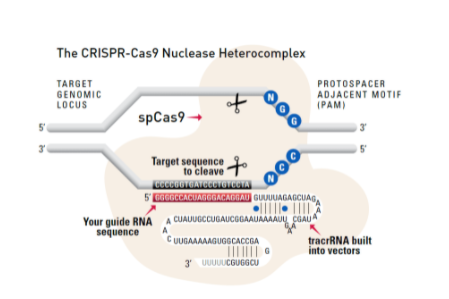
Cas9 protein—uses guide RNA (gRNA) to direct site-specific, double-strand DNA cleavage adjacent to a protospacer adapter motif (PAM) in the target DNA.
gRNA—RNA sequence that guides Cas9 to cleave a homologous region in the target genome. Efficient cleavage only where the gRNA homology is adjacent to a PAM.
PAM—protospacer adapter motif, NGG, is a target DNA sequence that spCas9 will cut upstream from if directed to by the gRNA.
The workflow at-a-glance
DESIGN: Select gRNA and HR donor plasmids. Choice of gRNA site and design of donor
plasmid determines whether the homologous recombination event results in a knock-out,
knock-in, edit, or tagging.
CONSTRUCT: Clone gRNA into all-in-one Cas9 vector. Clone 5’ and 3’ homology arms into HR
donor plasmid. If creating a knock-in, clone desired gene into HR donor.
CO-TRANSFECT or CO-INJECT: Introduce Cas9, gRNA, and HR Donors into the target cells
using co-transfection for plasmids, co-transduction for lentivirus, or co-injection for mRNAs.
SELECT/SCREEN: Select or screen for mutants and verify.
VALIDATE: Genotype or sequence putative mutants to verify single or biallelic conversion.
Supporting Data
Validating SBI’s CRISPR/Cas9 SmartNickase and Null Nuclease Vectors
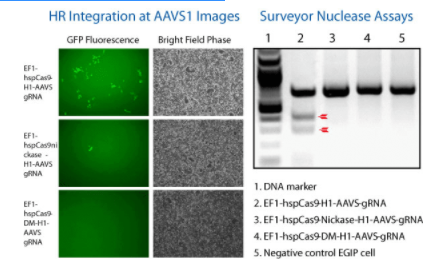
Figure 1. The All-in-one Cas9 Null Nuclease Vector shows no genome editing activity. We compared the activity of wild-type Cas9 (EF1α-hspCas9-H1-gRNA All-in-one SmartNuclease Plasmid, Cat.# CAS900A-1), Cas9 SmartNickase (EF1α-hspCas9-nickase-H1-gRNA All-in-one SmartNickase Plasmid, Cat.# CAS800A-1), and Cas9 Null Nuclease (EF1α-hspCas9-DM-H1-gRNA All-in-one Cas9 Null Nuclease Plasmid, Cat.# CAS805A-1) to insert a GFP reporter at the AAVS1 site through the use of AAVS1-targeting gRNA and an AAVS1-targeting HR Targeting Vector (AAVS1 Safe Harbor cDNA/miRNA Targeting HR Donor Vector (pAAVS1D-PGK-MCS-EF1α-copGFPpuro), Cat.# GE602A-1).(Left panel) Fluorescence (left-most column) and bright field (right-most column) microscopy showing that both Cas9 SmartNuclease (top set) and Cas9 SmartNickase (middle set) are able to insert GFP into the genome, whereas the Cas9 Null Nuclease is not (bottom set).
(Right panel) A Surveyor Nuclease Assay corroborates the lack of genome editing capabilities of the Cas9 Null Nuclease. The lack of cleavage seen in the SmartNickase lane is likely due to the lower frequency of GFP insertion.