Ironii Nitrate Formula
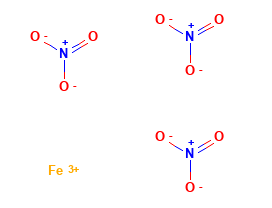
The formula of iron(III) nitrate is Fe(NO3)3. It is a pale violet crystalline solid that is soluble in water. Iron(III) nitrate is a strong oxidizing agent and can react with a variety of materials, including metals, organic compounds, and reducing agents.
Iron(III) nitrate is used in a variety of applications, including:
- Water treatment: Iron(III) nitrate is used as a coagulant and flocculant to remove suspended solids from water.
- Fertilizer: Iron(III) nitrate is used as a fertilizer to provide iron to plants.
- Pigments: Iron(III) nitrate is used to produce a variety of pigments, including yellow, brown, and red iron oxides.
- Other uses: Iron(III) nitrate is also used in a variety of other applications, such as:
- Wood preservation
- Leather tanning
- Photography
- Medicine
Iron(III) nitrate is a versatile and important chemical compound with a wide range of applications.
Here is a chemical equation for the preparation of iron(III) nitrate from iron and nitric acid:
Fe + 4 HNO3 → Fe(NO3)3 + NO + 2 H2O
Iron(III) nitrate is a hazardous material and should be handled with care. It is corrosive and oxidizing, and it can react violently with a variety of materials. It is important to wear appropriate personal protective equipment (PPE) when handling iron(III) nitrate.
