System Biosciences
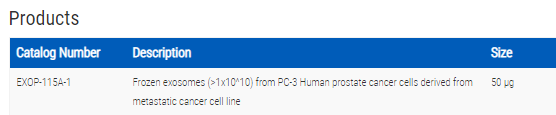
PC-3 Human prostate cancer cells derived from metastatic cancer cell line: >1x10^10 frozen exosomes
- SKU:
- EXOP-115A-1
- Availability:
- Usually Shipped in 5 Working Days
- Size:
- 50 ug
- Shipping Temperature:
- Dry Ice
Description
PC-3 Human prostate cancer cells derived from metastatic cancer cell line: >1x10^10 frozen exosomes. Cat# EXOP15A. Supplier: SBI System Biosciences
- Highly pure
- Ready-to-use
- Well-characterized
- Fully functional
Overview
- Highly pure
- Ready-to-use
- Well-characterized
- Fully functional
Supporting Data
Validated using Western blotting, NanoSight Analysis
All exosome preps are checked for the presence of CD63, a common exosome marker, via Western blotting (Figure 1).

Figure 1. All exosome preps contain CD63. Aliquots of purified exosomes from the cell lines and from human serum were lysed with either RIPA or M-PER buffer to make exosome protein lysates. Approximately 20 ug of protein for each sample was separated on a gradient SDS-PAGE and then transferred to nitrocellulose membranes. The membranes were probed for CD63 profiles using SBI’s anti-CD63 antibody (cat# EXOAB-CD63A-1) at a 1:1,000 dilution. Bands were detected using the secondary HRP-conjugated antibody at 1:10,000 and blots imaged. All purified exosome preparations were positive, immunoreactive for CD63 with the expected, variable banding patterns common to published exosome CD63 profiles.
All exosome preps are also subjected to NanoSight Analysis to test for particle size and intactness (Figure 2).

Figure 2. All exosome preps are checked for particle size and intactness via NanoSight. Approximately 5 µl of purified exosomes were added to 995 µl of 0.2 µm filtered 1X PBS (1:200 dilution). The diluted samples were incubated in a VWR 500 model ultrasonicator water bath set at 33°C for 10 minutes to ensure adequate exosome particle dispersion. The samples were diluted 1:10 then vortexed at 2.5k for 10 seconds. This eventual 1:2,000 dilution was used to gather between 1,000 to 3,000 particle tracks per sample analysis. The samples were then loaded into a NanoSight LM10HSB with a syringe pump and the sensitivity of the camera is set to auto 16 (the most sensitive auto-setting). All data were collected in triplicate. The purified exosomes displayed the expected size distribution profiles, with peak diameters from 90 – 110 nm and concentrations in the range expected for media exosomes at about 1 x 1010 exosomes/ml.















